Abstract
Background: Hemophilia inhibitors are associated with increased morbidity, mortality and health care costs. Although inhibitors are adverse reactions to pharmaceutical products, it is likely that they are under-reported. Data are needed on the burden of inhibitors in the U.S. to aid in prevention efforts.
Methods: The Centers for Disease Control and Prevention (CDC) integrated centralized inhibitor testing along with extensive inhibitor data collection as part of a public health surveillance system called Community Counts established in the USHTCN beginning in December 2013. HTC staff collect data on demographic (e.g., age, sex, race and ethnicity) and clinical (e.g., hemophilia type, severity, prescribed treatment) characteristics at annual comprehensive visits using standardized data forms. Data collected on inhibitors include the dates of first detection for any participants with a previous inhibitor history, results of the most recent local inhibitor test and changes in treatment such as use of bypassing agents or immune tolerance therapy. For the surveillance or for central lab confirmation of a locally identified recent new-onset elevated inhibitor titer, a blood sample is prepared and shipped to CDC where it undergoes testing using a validated modified Nijmegen Bethesda assay as previously described (Miller CH, et. al., JTH, 2012). Newly detected inhibitor titer elevations either from local testing (above lab threshold) or from CDC testing (≥0.5 NBU or ≥0.3 NBU for hemophilia A (HA) or B (HB), respectively) were identified prospectively and tests were repeated with a new blood specimen for confirmation. Specimens tested at CDC with titers above the cutoff but below 2 NBU received further testing to rule out false positive tests. Additional participants with a recent history of first elevated inhibitor titer within the 3 year surveillance period were also identified retrospectively; historical and treatment information and central lab testing were used to confirm the elevated titer. Participants with a new-onset elevated titer confirmed by at least one elevated titer by CDC testing and no reported history of an inhibitor prior to December 2013 were considered cases for surveillance purposes. The characteristics of the cases according to subgroups based on age and hemophilia type and severity are shown as the proportion of those with elevated titers among all participants tested in the subgroup with no previous inhibitor history.
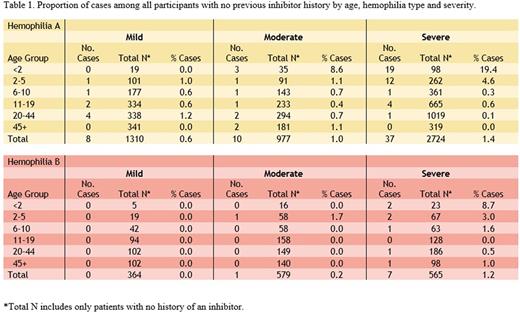
Results: During the period December 15, 2013 through December 31, 2016, a total of 7,718 males with HA or HB were enrolled in the surveillance, approximately 27% of hemophilia patients seen in the USHTCN. At enrollment 1124/6135 HA and 75/1583 HB patients had a history of inhibitor for a baseline inhibitor prevalence of 18.3% and 4.7% for HA and HB, respectively (including all severities). During the study period there were 55 cases of confirmed new-onset elevated inhibitor titers among 5,011 HA and 8 cases among 1,508 HB participants for overall proportions of 1% and 0.5%, respectively. Table 1 shows the proportions of cases among all participants with no inhibitor history by age, type and severity subgroup. As expected, the highest proportions of new cases were observed among young children with severe HA, totaling 8.6% in children < 6 years, compared to 4.4% in severe HB. Notable findings include: 1) 3.2% cases in children < 6 years with moderate HA; 2) 8.7% cases in severe HB < 2 years; 3) in participants > 5 years, cases comprised 0.25% and 0.63% of participants with severe HA and HB, respectively; and 4) cases beyond age 5 were observed in all age groups and severities of HA while new cases were identified in 0/364 mild and only 1/579 moderate HB participants.
Conclusions: These preliminary data provide the first measure of the burden of inhibitors among the U.S. population receiving care in the USHTCN. Sub-analyses for race, ethnicity and other risk factors will be possible when the data set matures. A prospective study of a large cohort was able to identify inhibitor risk in rare populations including participants who are > 5 years with HB or with moderate and mild HA or HB. These data will be useful in targeting populations for research and evaluating the effectiveness of public health prevention programs.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Sharathkumar: CSL Behring: Consultancy; Shire: Consultancy. Kempton: Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal